 xMas Holidays
xMas Holidays
Aptar’s Nasal Unidose System to Deliver neffy® (epinephrine nasal spray)
AptarGroup, Inc. (NYSE: ATR), a global leader in drug and consumer product dosing, dispensing and protection technologies, today announced that its Unidose Liquid System (Unidose) is the delivery system approved with neffy® (epinephrine nasal spray), the first and only needle-free treatment approved by the U.S. FDA for the emergency treatment of patients with allergic reactions (Type I), including anaphylaxis. This marks the first regulatory approval worldwide for nasally-delivered epinephrine.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20240815936474/en/
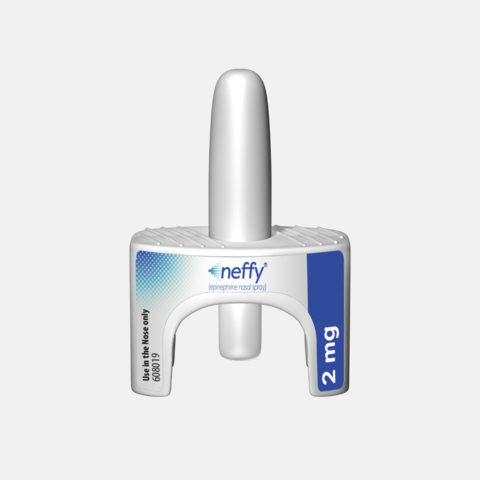
Photo: Aptar’s Unidose System for the neffy® 2 mg (epinephrine nasal spray). Image courtesy of ARS Pharmaceuticals.
Aptar’s Unidose is a single-use, ready-to-use, one-step nasal delivery system used to administer neffy to a patient during a severe allergic reaction. During such an event, the patient, healthcare professional, caregiver or user presses a small plunger on the bottom of the nasal spray system to release the drug in a single spray into the nostril.
Stephan B. Tanda, Aptar President and CEO, stated, “Aptar has been a leader in nasal delivery of medication for more than 30 years. We are proud of our role in the pharma industry to increase the use of nasally delivered medications that help promote adherence and ease of use for patients.”
Unidose (UDS) and Bidose (BDS) Technology Platforms
Aptar’s UDS and BDS technology platforms are designed to be robust, reliable and intuitive systems for easy administration by patients or caretakers. These drug delivery systems are designed and manufactured with strict quality controls intended to meet FDA’s guidelines for reliability. They offer biotech and pharmaceutical companies effective and reliable single or two-shot intranasal delivery for a variety of medicines including for emergency use and treatments of severe conditions. They can also be integrated with wireless connectivity technologies.
Accelerated Development Support via Aptar Pharma Services
This novel treatment for severe allergic reaction is an example of a Combination Product submission, and benefited from Aptar Pharma’s Services offering, a comprehensive portfolio of stage-specific development packages. Aptar’s dedicated Regulatory Affairs experts and analytical scientists help customers proactively address regulatory needs to help accelerate approval.
“The approval of neffy, which uses our Unidose System, and is the first nasally-delivered epinephrine treatment for severe allergic reaction, including anaphylaxis, once again demonstrates Aptar Pharma’s ‘formulation to patient’ focus on helping our customers develop complex, innovative treatments,” stated Gael Touya, President, Aptar Pharma. “When we combine our nasal systems’ capabilities with our Aptar Pharma Services offering, we bring added value to our customers, and aim to provide further convenience for patients and their caregivers worldwide.”
About Aptar
Aptar Pharma is part of AptarGroup, Inc., a global leader in drug and consumer product dosing, dispensing and protection technologies. Aptar serves a number of attractive end markets including pharmaceutical, beauty, food, beverage, personal care and home care. Using market expertise, proprietary design, engineering and science to create innovative solutions for many of the world’s leading brands, Aptar in turn makes a meaningful difference in the lives, looks, health and homes of millions of patients and consumers around the world. Aptar is headquartered in Crystal Lake, Illinois and has over 13,000 dedicated employees in 20 countries. For more information, visit www.aptar.com.
About ARS Pharmaceuticals
ARS is a biopharmaceutical company dedicated to empowering at-risk patients and caregivers to better protect patients from severe allergic reactions that could lead to anaphylaxis. The company is commercializing neffy®, an epinephrine nasal spray product for patients with Type I allergic reactions, including food, medications and insect bites that could lead to life-threatening anaphylaxis. For more information, visit www.ars-pharma.com.
This press release contains forward-looking statements. Forward-looking statements generally can be identified by the fact that they do not relate strictly to historical or current facts and by use of words such as “expects,” “anticipates,” “believes,” “estimates,” “future,” “potential,” “continues” and other similar expressions or future or conditional verbs such as “will,” “should,” “would” and “could” are intended to identify such forward-looking statements. Forward-looking statements are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on our beliefs as well as assumptions made by and information currently available to us. Accordingly, our actual results or other events may differ materially from those expressed or implied in such forward-looking statements due to known or unknown risks and uncertainties that exist in our operations and business environment including, but not limited to: the successful integration of acquisitions; the regulatory environment; and competition, including technological advances. For additional information on these and other risks and uncertainties, please see our filings with the Securities and Exchange Commission, including the discussion under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Form 10-K and Forms 10-Q. We undertake no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as otherwise required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20240815936474/en/
Aptar Investor Relations Contact:
Mary Skafidas
+1 347 351 6407
mary.skafidas@aptar.com
Aptar Pharma Media Contact:
Carolyn Penot
+33 6 37 36 76 84
carolyn.penot@aptar.com
Aptar Media Contact:
Katie Reardon
+1 815 479 5671
katie.reardon@aptar.com