Paragon 28 Launches the Phantom® Fibula Nail System for Less Invasive Fracture Repair
Paragon 28, Inc. (NYSE: FNA) is pleased to announce the launch of the Phantom® Fibula Nail System, designed to give surgeons a less invasive option to treat the fibula when patients sustain an ankle fracture. Use of a fibula nail for the treatment of ankle fractures has been shown to have significantly fewer soft tissue complications, implant removals, and fracture nonunion when compared to plate and screw fixation, a common alternative approach.1
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241016226468/en/
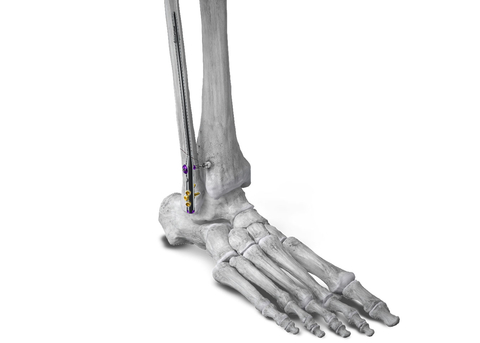
Figure 1: Phantom® Fibula Nail implanted in the fibula for fracture stabilization with syndesmotic repair using the R3FLEX™ Stabilization System (Photo: Business Wire)
The Phantom® Fibula Nail System provides surgeons with instrumentation to help facilitate anatomic reduction and proper implant placement. This includes an Entry K-Wire Guide to aid identification of the correct starting point for the nail and a Curved Reamer Guide to help direct the surgeon to the correct angle of the fibula at which to implant the nail. The system instrumentation also allows the surgeon to pull traction on the fibula to reduce the fracture intraoperatively. Innovative implant features such as screws that thread into the nail and an expandable proximal locking mechanism allow for optimal stability in the fibula. Additionally, syndesmotic fixation can be achieved through the nail with one of the many novel Paragon 28 syndesmotic implant options, including the Company’s recently launched R3FLEX™ and R3ACT™ Stabilization Systems.
Surgeon Designer Dr. Charles Moon comments, “The Phantom Fibula Nail System offers surgeons an excellent surgical option for treating a fractured fibula with several next-generation features that truly set the system apart. The novel instruments designed to provide a reproducible starting point and the ability to pull traction for fracture alignment add measurable value to surgeons and lead to better outcomes for patients. The threaded distal screws and user-friendly proximal locking option help optimize stability, which is vital to fracture healing.”
“I’m thrilled for patients and surgeons alike to experience the impact of the Phantom Fibula Nail System,” says Albert DaCosta, CEO of Paragon 28. “We’ve developed a special product here, with a design that sets it apart from current offerings for fibula fractures. The Phantom® Fibula Nail System gives surgeons precision and control for fractures that are often difficult to stabilize, which we expect will result in better, more reproducible patient outcomes.”
The Phantom® Fibula Nail System is delivered in one surgical tray along with a small set of sterile packed implants designed to simplify surgical workflow and inventory management.
The Phantom® Fibula Nail System adds to Paragon 28’s innovative foot and ankle fracture portfolio which includes the Gorilla® Ankle Fracture System, Gorilla® Pilon Plating System, Baby Gorilla® Plating System, Gorilla® Calcaneus Fracture Plating System, Gorilla® LisFranc Plating System, Monkey Bars™ External Fixation System, and PRECISION® Jones Fracture Screw System.
About Paragon 28, Inc.
Based in Englewood, CO., Paragon 28 is a leading medical device company exclusively focused on the foot and ankle orthopedic market and is dedicated to improving patient lives. From the onset, Paragon 28® has provided innovative orthopedic solutions, procedural approaches and instrumentation that cover a wide range of foot and ankle ailments including fracture fixation, forefoot, ankle, progressive collapsing foot deformity (PCFD) or flatfoot, Charcot foot and orthobiologics. The company designs products with both the patient and surgeon in mind, with the goal of improving outcomes, reducing ailment recurrence and complication rates, and making the procedures simpler, consistent, and reproducible.
Forward Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to: Paragon 28’s potential to shape a better future for foot and ankle patients. You are cautioned not to place undue reliance on these forward-looking statements. Forward-looking statements are only predictions based on our current expectations, estimates, and assumptions, valid only as of the date they are made, and subject to risks and uncertainties, some of which we are not currently aware. Forward-looking statements should not be read as a guarantee of future performance or results and may not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. These forward-looking statements are based on Paragon 28’s current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Paragon 28’s business in general, see Paragon 28’s current and future reports filed with the Securities and Exchange Commission, including its Annual Report on Form 10-K/A for the fiscal year ended December 31, 2023 and its Quarterly Reports on Form 10-Q, as updated periodically with its other lings with the SEC. These forward-looking statements are made as of the date of this press release, and Paragon 28 assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law.
Disclaimer
Dr. Moon may report consulting and royalty fees from Paragon 28 in connection with the provision of product development services to Paragon 28.
Nothing in this material is intended to provide specific medical advice or to take the place of written law or regulations.
References
- Tas, David B., et al. "Intramedullary fixation versus plate fixation of distal fibular fractures: a systematic review and meta-analysis of randomized controlled trials and observational studies." The Journal of Foot and Ankle Surgery 58.1 (2019): 119-126.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241016226468/en/
Contact
Investor Contact
Matthew Brinckman
Senior Vice President, Strategy and Investor Relations
Phone: (720) 912-1332



